Students may know many examples of everyday acids and bases from vinegar to bleach. However students may not know that acids and bases have different strengths. The strengths of acids and bases are illustrated on the pH scale. This demonstration is a very visual method of presenting the pH scale.
Video: The formulation of an acidic solution using dry ice
Methodology
Materials
- Universal indicator (liquid)
- Dry ice
- Sodium hydroxide
- 1L graduated cylinder
- Plastic dropper
- Water
Method
Fill up about 600ml of water. Add a few drops of universal indicator. Add a few drops of sodium hydroxide. Stir to ensure a homogenous mixture. Add dry ice to the mixture.
Tips
It is recommended that the dry ice is bought as dry ice made from a carbon dioxide cylinder attachment will float and is less effective at saturating the solutions. Dry ice can be obtained from universities, higher education institutions, hospitals and industry. It can be stored and transported in polystyrene boxes.
*Precautions/Safety
Theory behind the hook
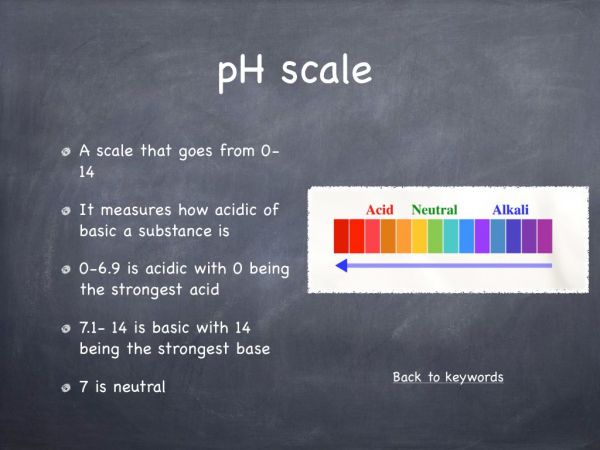
The pH of a substance can be measured by using a chemical called universal indicator. The pH scale goes from one to fourteen, one to seven is acidic, seven is neutral and from seven to fourteen is basic. Universal indicator is green in the water, which indicates that it is a neutral solution. When a base such as sodium hydroxide is added it turned into a purple colour. This illustrates that the solution has turned basic and it is roughly pH 12. When dry ice is added to the solution, a number of colour changes happen. These colour changes illustrate the different pHs that are present due to the addition of dry ice. When carbon dioxide is added to an aqueous solution, carbonic acid is formed. This is why the solution changes from a basic solution to an acidic solution. This is represented by the red colour observed at the end of the experiment.
H2O + CO2  = H2CO3
Water + Carbon dioxide = Carbonic acid
How this hook works
While this hook introduced the students to the pH scale using acids, bases and dry ice, it also reinforces a topic the students may have already covered. When discussing the characteristics of carbon dioxide, the students learn that carbon dioxide dissolves in water to form an acidic solution. This has proven difficult for the students to understand in the past. This demonstration should help the students remember that carbon dioxide forms an acidic solution in water by using it to illustrate the range of colours in the pH scale. The demonstration is highly visual and the use of dry ice makes the experiment fun and memorable. This hook could be conducted by the teacher during the topic of acids and bases when introducing the pH scale. The demonstration can take up to 15 min.
Questions & Answers
- Why does the pH increase when sodium hydroxide is added?
The pH increases when sodium hydroxide is added as it is a base and this causes the solution to turn basic. The pH of a basic solution if from 7-14. - Why does the pH decrease when the dry ice is added?
The pH decreases when dry ice is added because it forms an acid solution (carbonic acid) when it is added to water. The pH of an acidic solution is from 1-7. - If universal indicator is green in water what does that tell us about water?
The green colour of universal indicator tells us that water has a pH of 7 i.e. neutral. - What is the pH scale?
The pH scale tells us how acidic or basic a substance is. - Is there any other method of testing the pH?
The pH of a substance can be measured by using a pH meter.
Cross Curricular Links
Links can naturally be made to acids and bases in Leaving Certificate chemistry. This demonstration can also be linked to the geography syllabus when discussing acid rain and its effect on the environment. Acids damages teeth (enamel erosion) which could link to the food section in junior certificate science and home economics.