This video creates an amazing colour-changing liquid you can make with cabbage. The solution shows if a substance is an acid or base. When the cabbage solution is mixed with another liquid, a colour change occurs and indicates if the solution is acidic or basic. This is a fun, visual experiment that uses everyday objects.
Methodology
Materials
- Red cabbage
- Chopping board and knife
- Water
- A blender
- A sieve
- A measuring jug and spoon
- Various jars/glasses to hold liquids
- Household items to test such as: Milk, 7- Up, Water, Rennie, Baking Powder, Lemon, Mashed Kiwi, Vinegar, Fabric Softener, WashingUp Liquid, Bleach, Drain Cleaner.
Method
- Cut up about a quarter of a head of red cabbage into small pieces.
- Place the cabbage and about 250ml of water into a blender and blend until the cabbage is in uniform tiny pieces.
- Pour the mixture through the sieve pushing the liquid through with the back of a spoon if needed. The strained liquid is our indicator and it is what we will use to test our household liquids.
- Pour equal amounts of the household liquids into jars or glasses (it must be a clear container in order to see the colour change).
- Now pour a small amount of your strained liquid (the red cabbage indicator) into each of the household liquids.
- Take note of the colour change in each liquid and compare this with the Red Cabbage Indicator scale below in order to determine the pH of the liquid.
Precautions
- You must be careful to keep strong acids and bases well away from children.
- You must also warn students that they must never drink anything during an experiment as it can be dangerous.
- Some household products can cause skin irritations. Do not allow these to contact skin; rinse thoroughly with water if they do.
Teacher Note
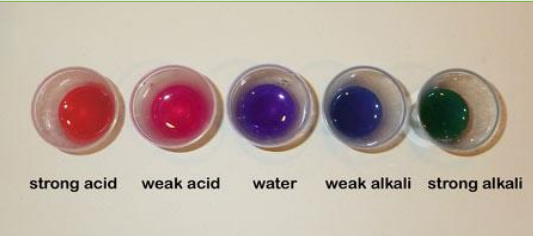
Some very common household solutions are acids and bases. Acids are solutions that will donate hydrogen ions in a solution, and usually taste sour. Some common acids are citrus fruit juices and household vinegar. Bases are solutions that accept hydrogen ions in solution, and usually feel slippery. How do you tell if something is an acid or a base? You use a chemical called an indicator, which changes in colour depending on whether a solution is acidic or basic (alkali). (Specifically, an indicator works by responding to the levels of hydrogen ions in a solution.) There are many different types of indicators, some are liquids and some are concentrated on little strips of “litmus” paper. Indicators can be extracted from many different sources, including the pigment of many plants. Red cabbage contains an indicator pigment molecule called flavin. Very acidic solutions will turn anthocyanin, a red colour. Neutral solutions result in a purplish colour. Basic solutions make a greenish-yellow or yellow colour. Because red cabbage has this indicator pigment, it is possible to determine the pH of a solution based on the colour it turns the red cabbage juice. The pH of a solution is a numerical measure Chemistry Kitchen of how basic or acidic it is. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid (see figure 1).
Extension Activities and Worksheets
This video experiment has four worksheets. The first is a results worksheet that students can fill out in class. The remaining three are the early, middle and upper year worksheets. Access to worksheets and extension activities are available in the teacher resource pack to Kitchen Chemistry.