This is largely a new topic for first year science students and is an introduction to the language of basic chemistry. To deepen the understanding of the students, this activity using paper clips can be used when introducing concepts and definitions from the topic. This activity could be revisited at the end of the topic in the form of an assessment
for learning strategy in which students are asked to explain the definitions using paper clips.
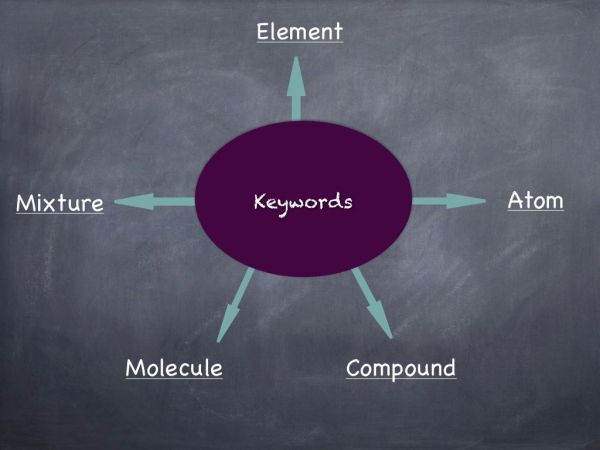
Video: Explanation of key chemistry terms using paper clips
Methodology
Materials
- Paper clips of various colours and sizes
Method
Divide paper clips into piles of the same size and colour-these are our elements. Take one pile of paper clips and divide that pile in two equal piles. Continue dividing piles until you are left with one single paper clip in each pile- this represents the atom, the smallest part of an element that has the properties of the element. Using a set of wire cutters or a pair of scissors, cut the paper clip into smaller pieces- no longer an atom. Link two papers clips that are the same size and colour in a “bondâ€- this represents a molecule. Link two or more paper clips that are different sizes or colours to create different molecules. Group these different molecules into a pile to create a mixture. Separate paper clips from the mixture based on the different linkages created. Join two different sets of linked paperclips together this represents a compound. Try to pull the paper clips apart to separate them.
Theory behind the hook
All of the elements on the periodic table are made of atoms: This is represented using the piles of paper clips. All atoms of a single element are the same: all the paper clips in the pile are the same size and colour. Atoms of different elements have different characteristics (size, properties) – different sizes and colours of paper clips. A molecule is made up of two or more atoms chemically combined: Two or more paper clips are joined or linked together in a ‘bond’. They are not as easily separated as a mixture. E.g. O2, H2O, CH4. Mixtures are made up of two or more substances mingled together but not chemically combined. A mixture of substances can be easily separated. Atoms of different elements can combine to form compounds: you can link different sizes and colours of paper clips together to make new structures. Compounds are more difficult to separate than mixtures. In chemical reactions, atoms are not made, destroyed, or changed. The paper clips were not destroyed, changed and no new paper clips created when the molecules and compounds were formed.
How this hook works
Elements, mixtures and compounds is a topic which largely introduces the students to the language of chemistry. The main learning point in the chapter is the definitions and terminology. This is the first time students will have learned about atoms, elements and compounds and from experience it seems most student rote learn the definition without much understanding. By representing elements as a pile of paper clips, most of the definitions can be introduced for this topic in a way which will promote understanding of the definitions. This demonstration can be used by the teacher at the start of the topic to introduce the terminology from this chapter and may be linked back throughout the chapter. The teacher may also decide to use this activity at the end of the topic by incorporating it into an assessment for learning strategy by asking the students to explain definitions using paper clips as reinforcement.
Questions & Answers
- What is the smallest unit present in this pile of paper clips?
One single paper clip. It is the smallest unit because it is the smallest repeating unit which makes up the group. It has the same characteristics of all other units in the pile. - How is this similar to an atom?
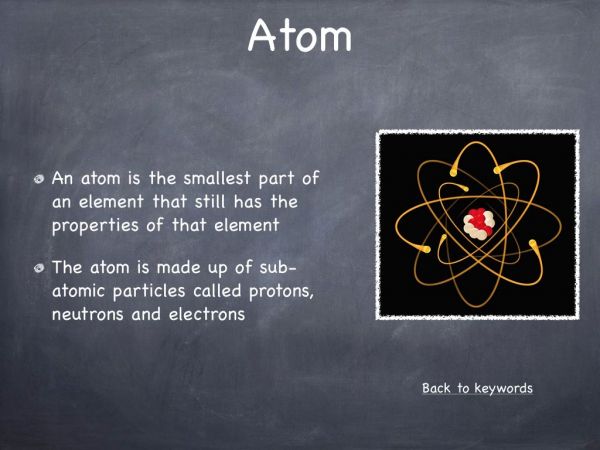
The atom is the smallest unit or part of an element. If the element was split into smaller and smaller pieces, we would eventually obtain single atoms. These atoms have the same properties and characteristics of the element. - Can an atom be broken into smaller particles?
Yes. An atom can be broken into smaller pieces called sub-atomic particles. Atoms in different elements have different numbers of sub-atomic particles. When the atom is broken up, however, it does not have the properties of the element (just like the paper clips no longer hold paper together when broken up). - What is an element?
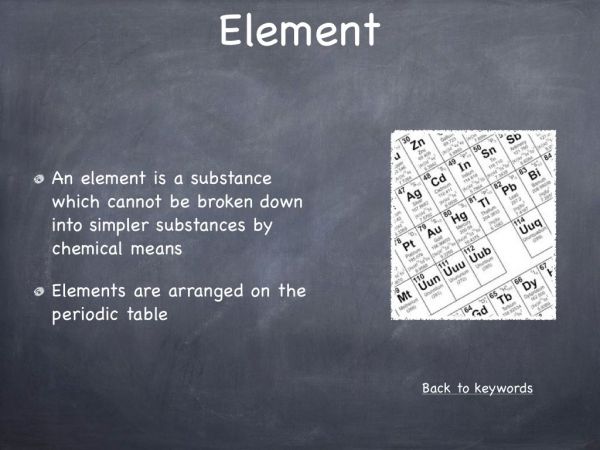
An element is a substance which cannot be broken down into simpler substances by chemical means. An element is made up of only one type of atom. - What is the difference between a molecule and a compound?
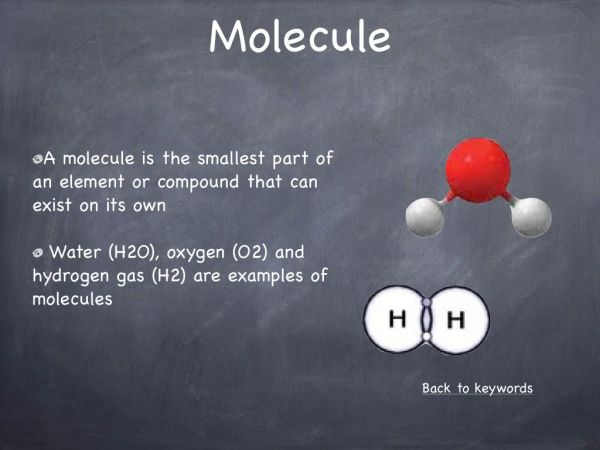
A molecule is the smallest part of an element or compound that can exist on its own e.g. O2. A compound has its own properties and is a completely new substance. A compound is formed when two or more elements chemically combine. The properties of a compound are different to those of the elements the compound is made of.
Cross Curricular Links
This activity links difficult concepts to a common paper clip. Learning can be extended with an introduction to the basic atomic structure when discussing the cutting of a paper clip into smaller particles. This activity could also be linked to separating mixtures.