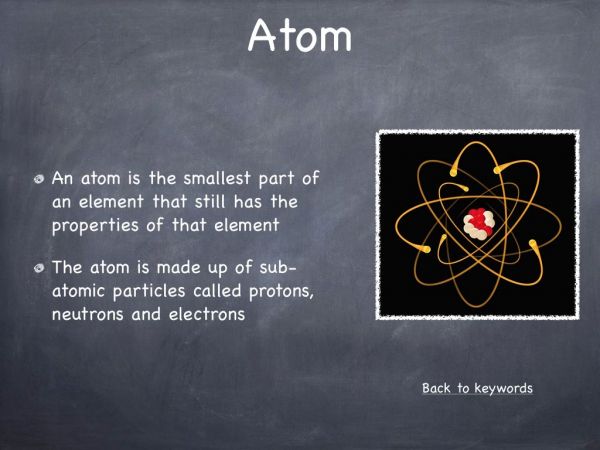
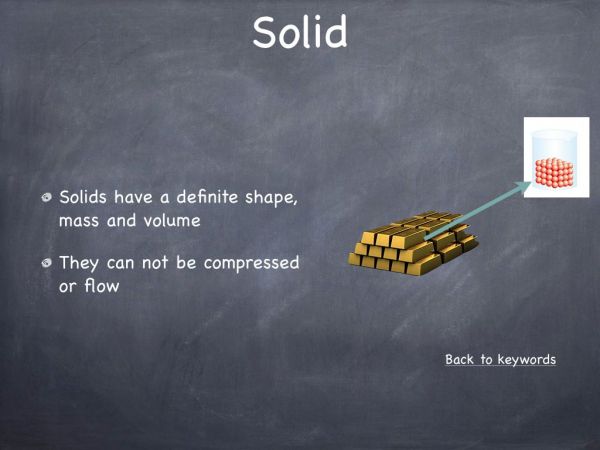
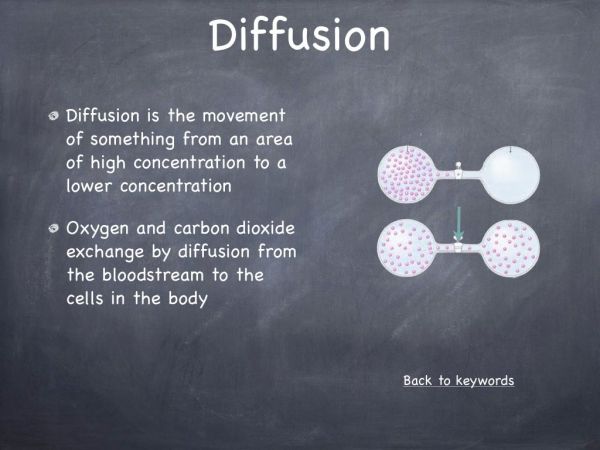
This experiment was designed to illustrate that a chemical reaction between a solid and a liquid can produce a gas. It is also a very visual representation, illustrating that gases occupy space and they move via diffusion. This experiment can be used to explain the arrangement of atoms in the three states of matter, solid, liquid and gas.
Video: Production of Carbon Dioxide
Methodology
Materials
- Bicarbonate of soda
- Vinegar
- Beaker/Jam jar
- Latex glove
Method
Add 2cm3 of vinegar to the beaker. Add three heap teaspoons bicarbonate of soda to the latex glove. Tightly stretch the glove over the top of the beaker, ensure no bicarbonate of soda is added at this point. Add all the bicarbonate of soda to the beaker.
Tips
To ensure the full expansion of the glove with gas a tight fitting glove is needed to stop any gas escaping.
Theory behind the hook
Bicarbonate of soda or baking soda is a base. Its more scientific name is a carbonate. Vinegar is an acid, the acid that is present in vinegar is called ethanoic acid. When acid and a carbonate are mixed together a chemical reaction takes place to produce salt, water and carbon dioxide. The carbon dioxide that is produced is a gas that is heavier than air. This gas pushes the air molecules up and thus the glove expands.
CH3COOH + NaHCO3 –> CH3COONa + H2O + CO2
Vinegar+ Bicarbonate of Soda –> Sodium acetate (salt) + Water + Carbon dioxide
How this hook works
The effectiveness of this hook lies in its simplicity. The students can carry out this experiment themselves and the materials involved are common household products. The gas inside the glove exerts a pressure, which enables the students to see the results of the experiment. This experiment can link numerous parts of the chapter from the formation of a gas from a solid and a liquid to the movement of gas.
Questions & Answers
- Why did the gas fill the entire glove?
Gases diffuse from an area of high concentration to an area of low concentration. - Why is carbon dioxide produce?
It is produced because there is a carbonate and an acid reacting together. - Why is it necessary for the glove to be tightly fitted?
The gas would diffuse out if there is any space.
Cross Curricular Links
This hook can be linked to acids and bases in relation to the reaction of an acid and carbonate to form salt water and carbon dioxide (acid + carbonate = Salt + water + carbon dioxide). The properties of carbon dioxide, the periodic table and bonding could also be discussed. With reference to Home Economics, the teacher could ask the students if they can come up with any reasons why bicarbonate of soda would be added to bread and cakes.